Better studies start with better design
With Faro, sponsors save time and improve quality, ensuring the highest-possible probability of success

Clinical development is complex - Faro helps sponsors balance complexity with operational reality to deliver excellent studies from beginning to end.
Better design, faster execution
Protocol design isn’t just a checklist— it’s the strategic backbone of your entire development plan. When you get it right, you reduce amendments, de-risk execution, and shave months off timelines.
Faro empowers study teams to design protocols that are both strategic and execution-ready, accelerating your whole program from the start.
Our approach helps sponsors meet their biggest goals
From small biotechs to global pharma enterprises, the world’s most innovative organizations are designing and digitizing their protocols with Faro.
Study Design
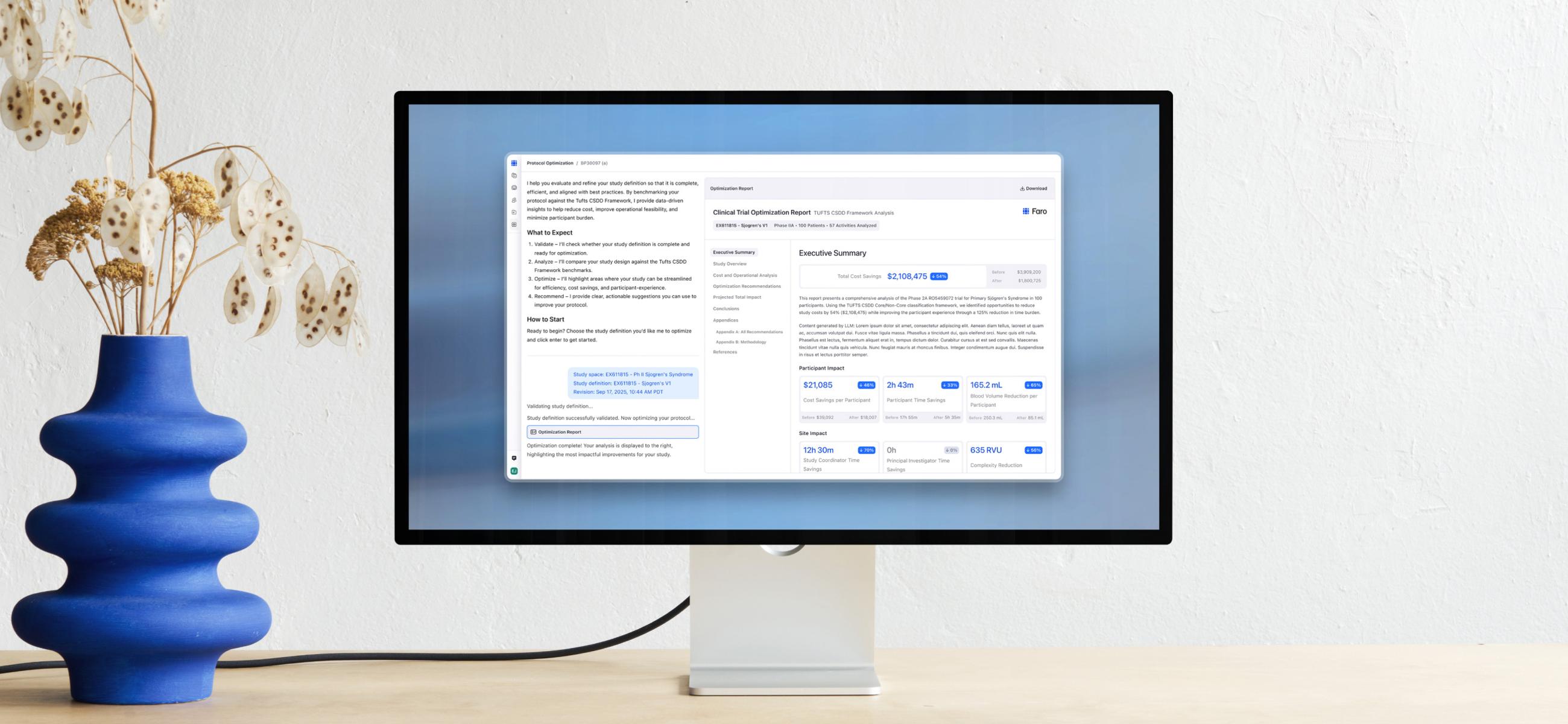
Optimize your clinical studies
With Faro’s AI-powered insights, study teams have all the data they need to inform collaborative decision-making, identify risks before they become problems, align faster across functions, and design optimal studies from the start.
Document Authoring
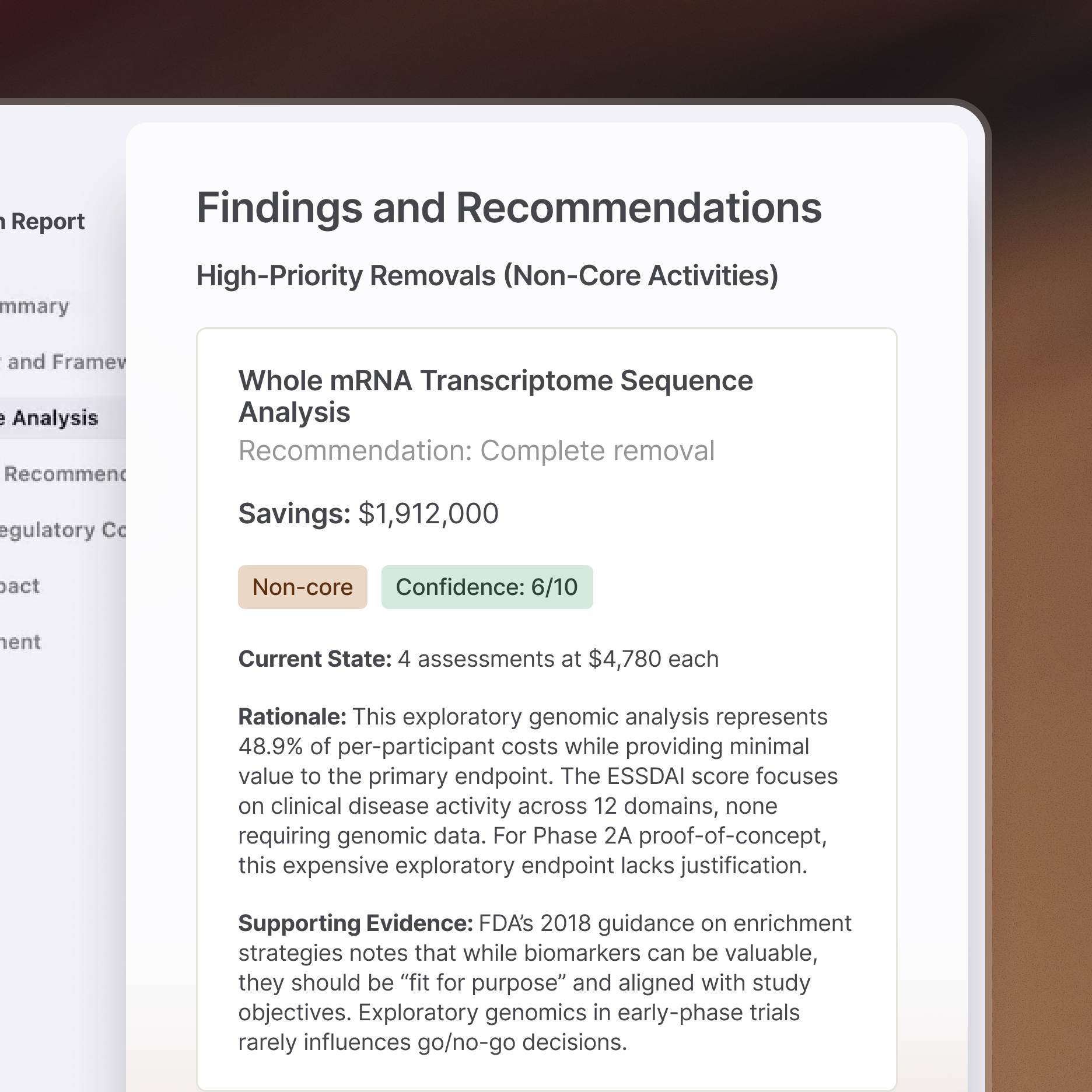
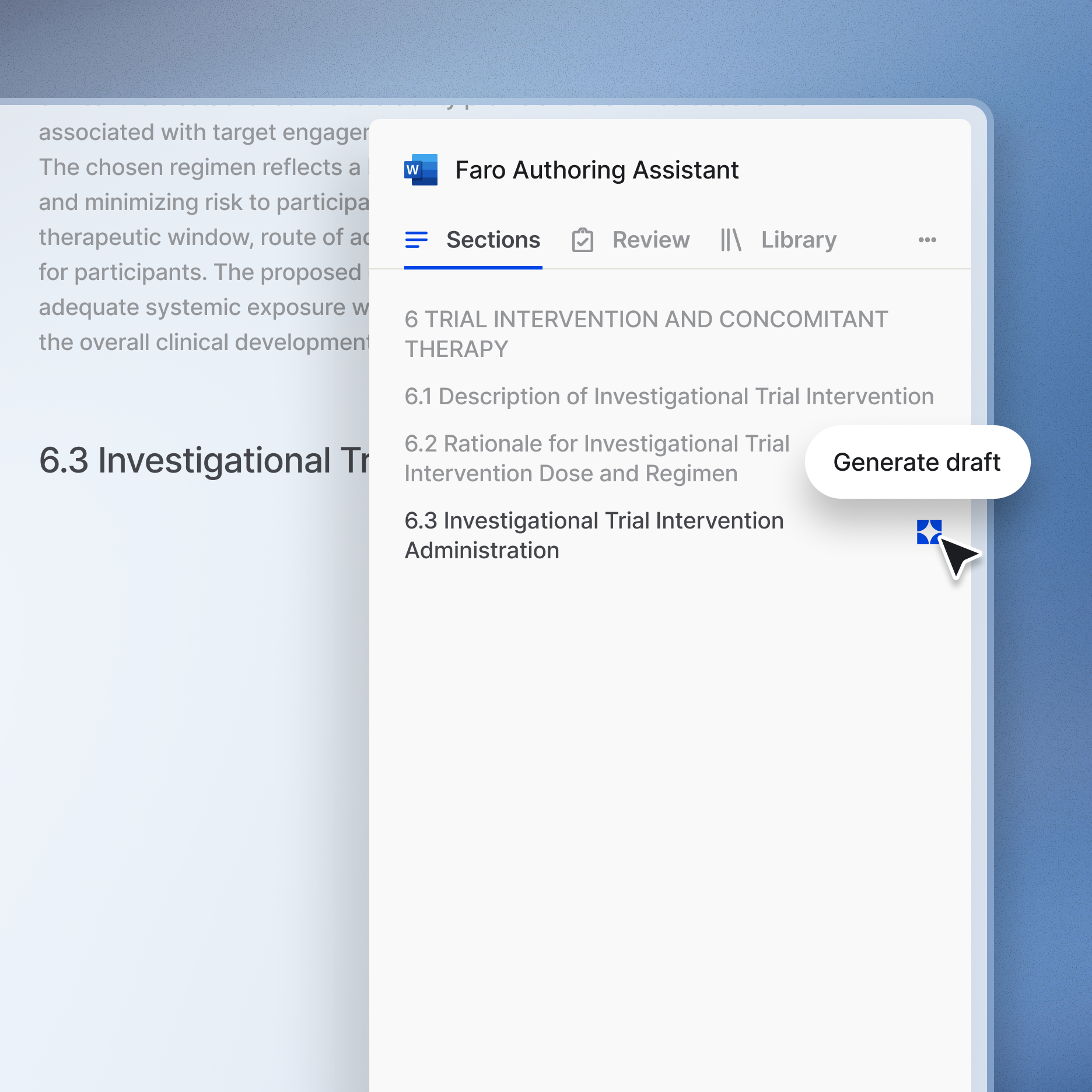
Accelerate clinical writing
Authoring is collaborative. Faro aligns clinical scientists and medical writers with a digital definition that supports clear communication and accelerates the drafting process.
Workflow Automation
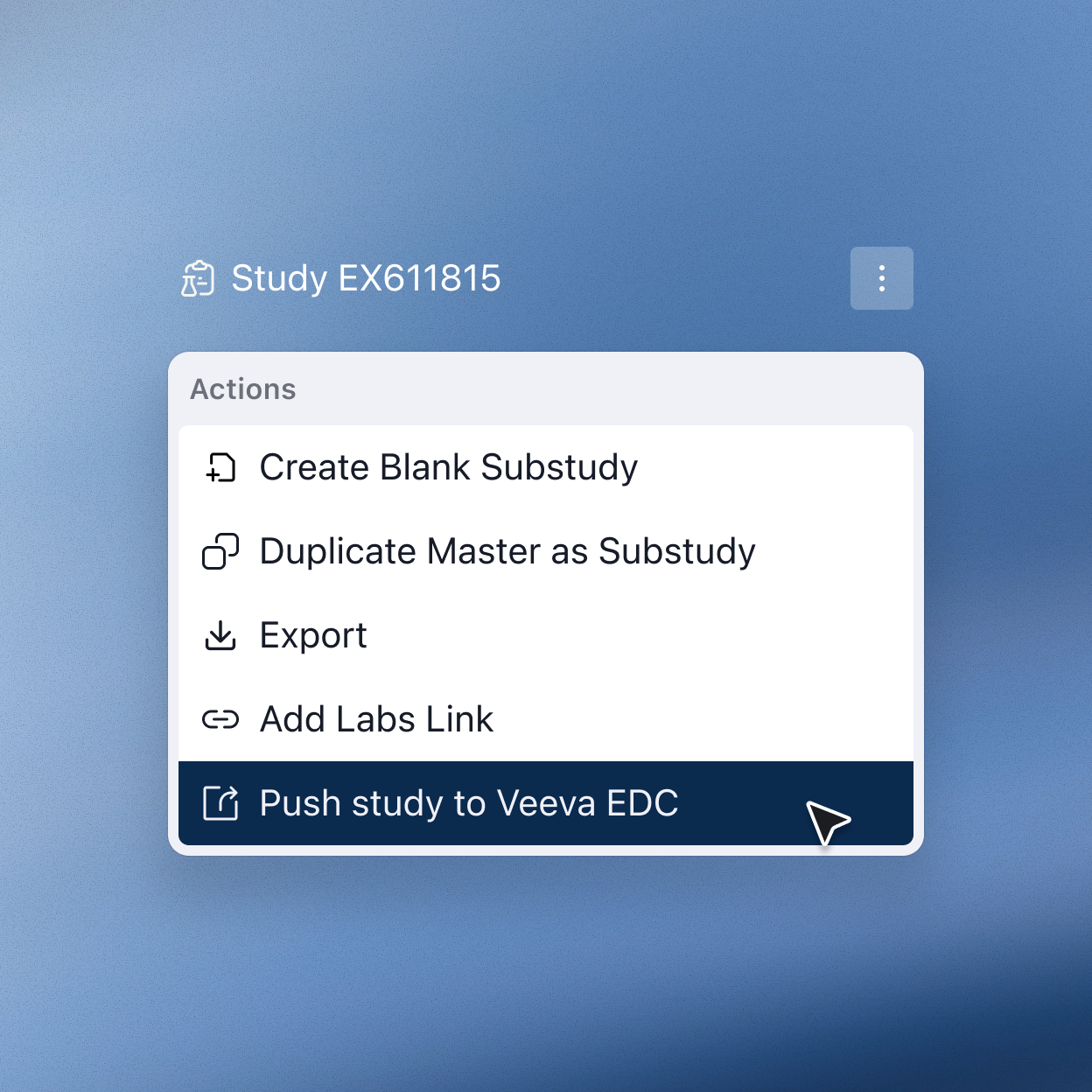
Accelerate downstream processes
With a digital study design, clinical teams have the power to reduce manual rework in downstream processes such as budgeting, EDC builds, and more — saving time and freeing them to focus on what matters most.
Unleash your protocol data using AI
With Faro, your whole clinical development team can realize the full value of digital data flow.
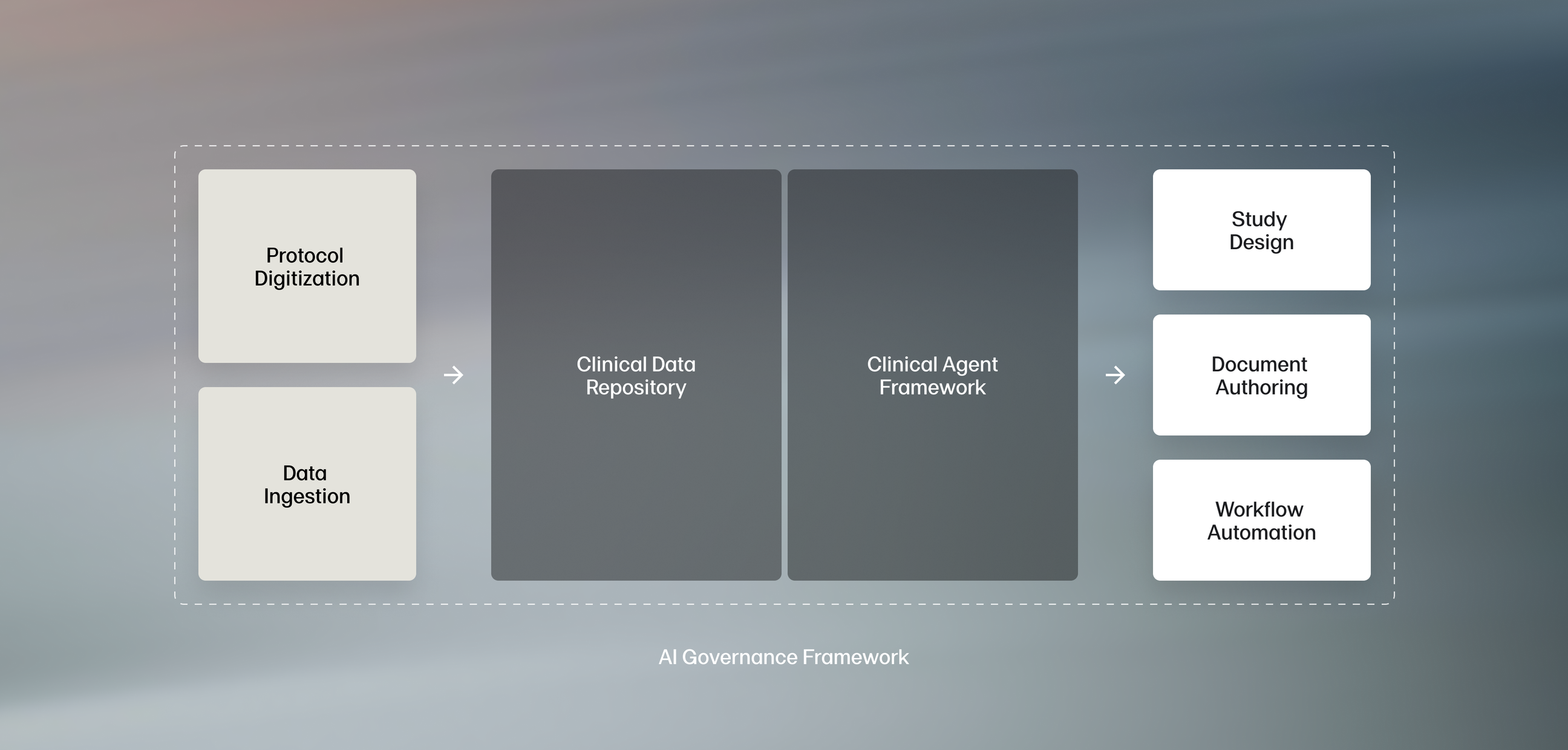
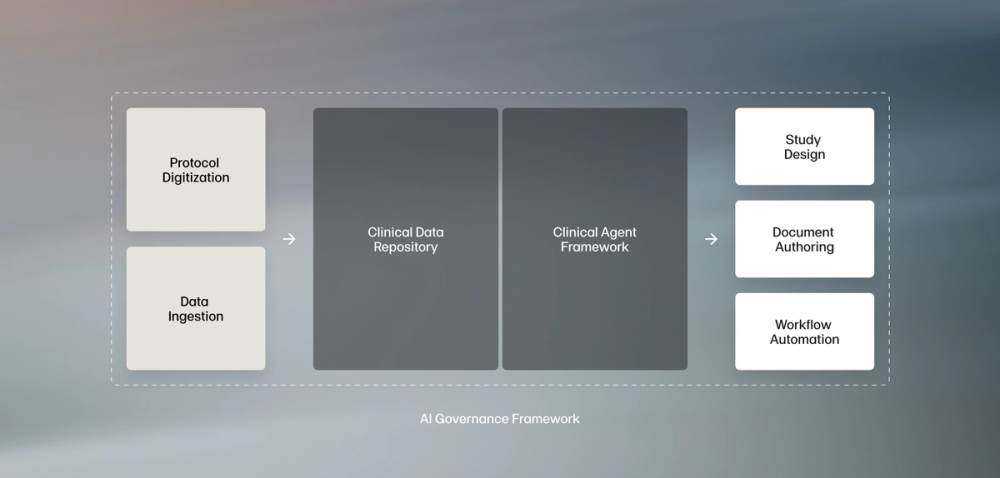
AI built specifically for clinical teams
Off-the-shelf AI isn't enough for the needs of life sciences sponsors. Faro's proven system is purpose-built for the needs of clinical development teams, with an intense focus on quality, accuracy, and compliance.
Instantly usable, out of the box
Faro's agentic systems are built by seasoned industry professionals, designed for the realities of clinical development, and integrate seamlessly into existing workflows from day one.
A powerful digital protocol repository
Faro's approach helps sponsors transform documents into data, providing the structured foundation that enables true AI-driven transformation.
Modern clinical trial development demands more than out-of the box LLMs
Faro’s agentic AI framework has been refined over years of hands-on deployment in top pharma and biotech environments. It combines deep clinical domain knowledge with operational awareness, translating decades of development experience into intelligent, context-aware support.
Built on experience
Our platform is grounded in deep domain expertise — from our in-house clinical experts and from practical experience helping top-20 pharma companies transform trial design and authoring.
Built for trust
Faro is built to the highest standards of security and compliance, never trained on your data, and ensures your proprietary information stays fully protected—always.
Built for quality
Faro applies a layered quality framework—combining expert human review with specialized AI validation—to ensure accuracy, completeness, and reliability, eliminating the common errors that generic language models introduce in complex clinical contexts.