Design, optimize, and start up trials more efficiently than ever, with powerful AI tools that bring a new level of insight and confidence to your process.
The industry’s first platform for digitally native trial design, our Study Designer is an intuitive workspace purpose-built to harness the complexity of modern clinical development. Faro is where smart, efficient, patient-friendly trials take shape—not just as high-quality documents, but as data that powers your whole program.

Plan robust, precise studies designed to succeed—quickly, predictably, and with fewer amendments and deviations.


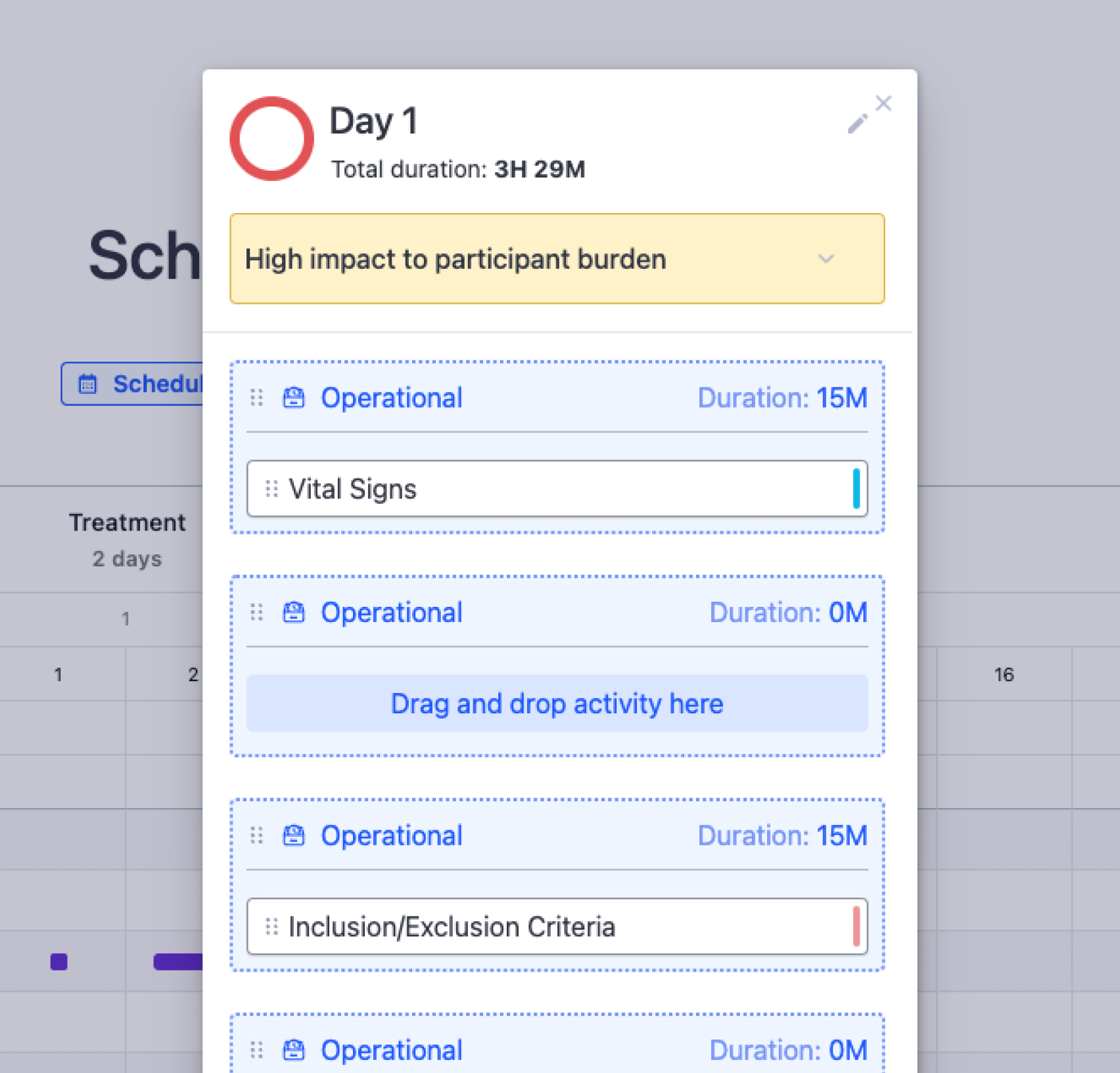
Become their sponsor of choice by using Faro to proactively mitigate executional challenges, eliminate feasibility hurdles, and lower burden to patient-friendly levels. Develop your trial with our Study Designer, and we’ll show you exactly how and where to de-risk your protocol, optimize it for smooth execution, and set sites up for successful recruitment.
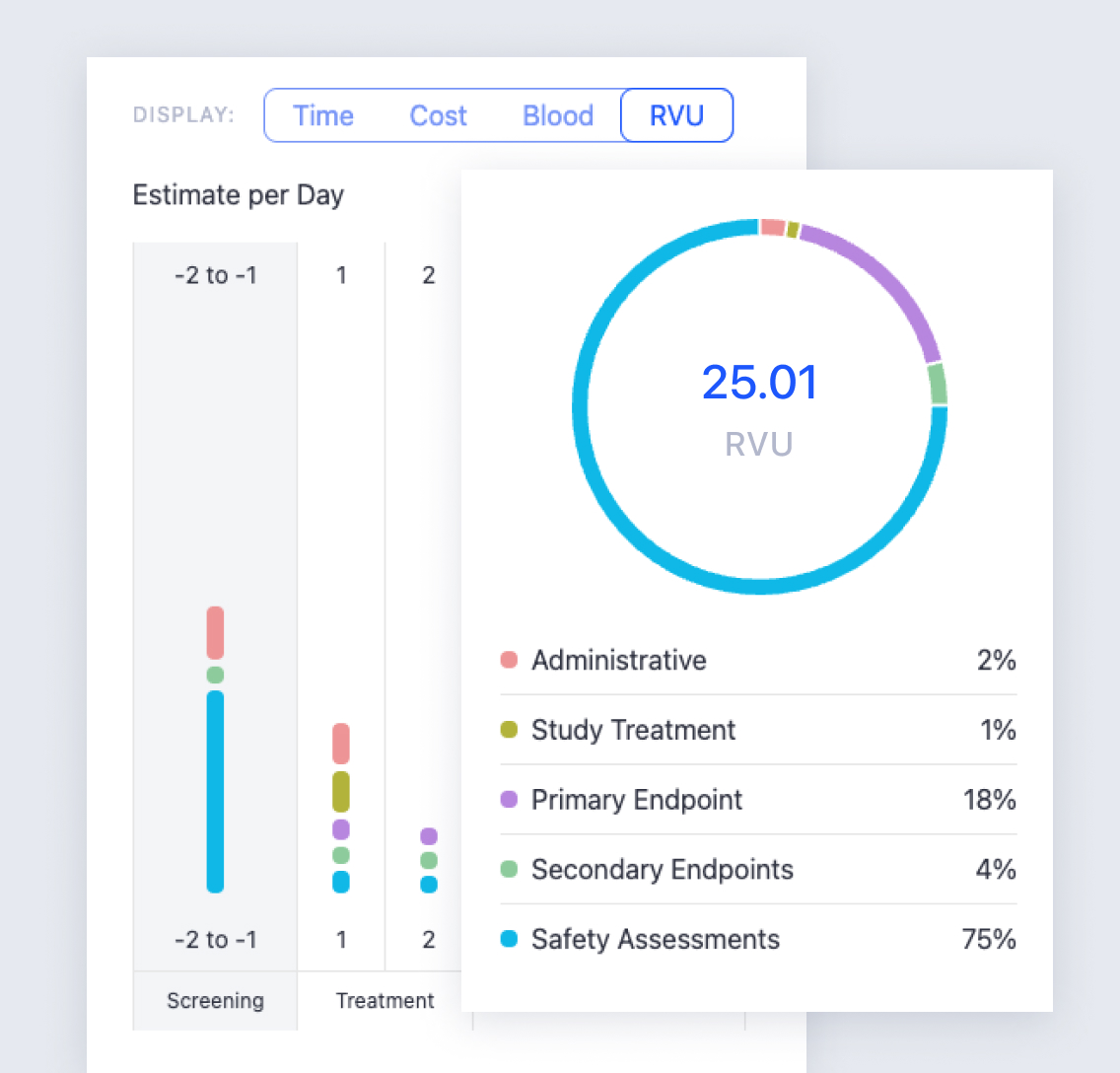
Say goodbye to attachments and email chains that put quality and consistency at risk. With the Faro Study Designer, your team can produce polished protocol content more seamlessly and collaboratively than ever.
In the Faro workspace, you can accelerate drafts with genAI tools, use smart content modules to maximize consistency, and keep your whole team aligned with a single shared view of your protocol—no emails or regroups required.
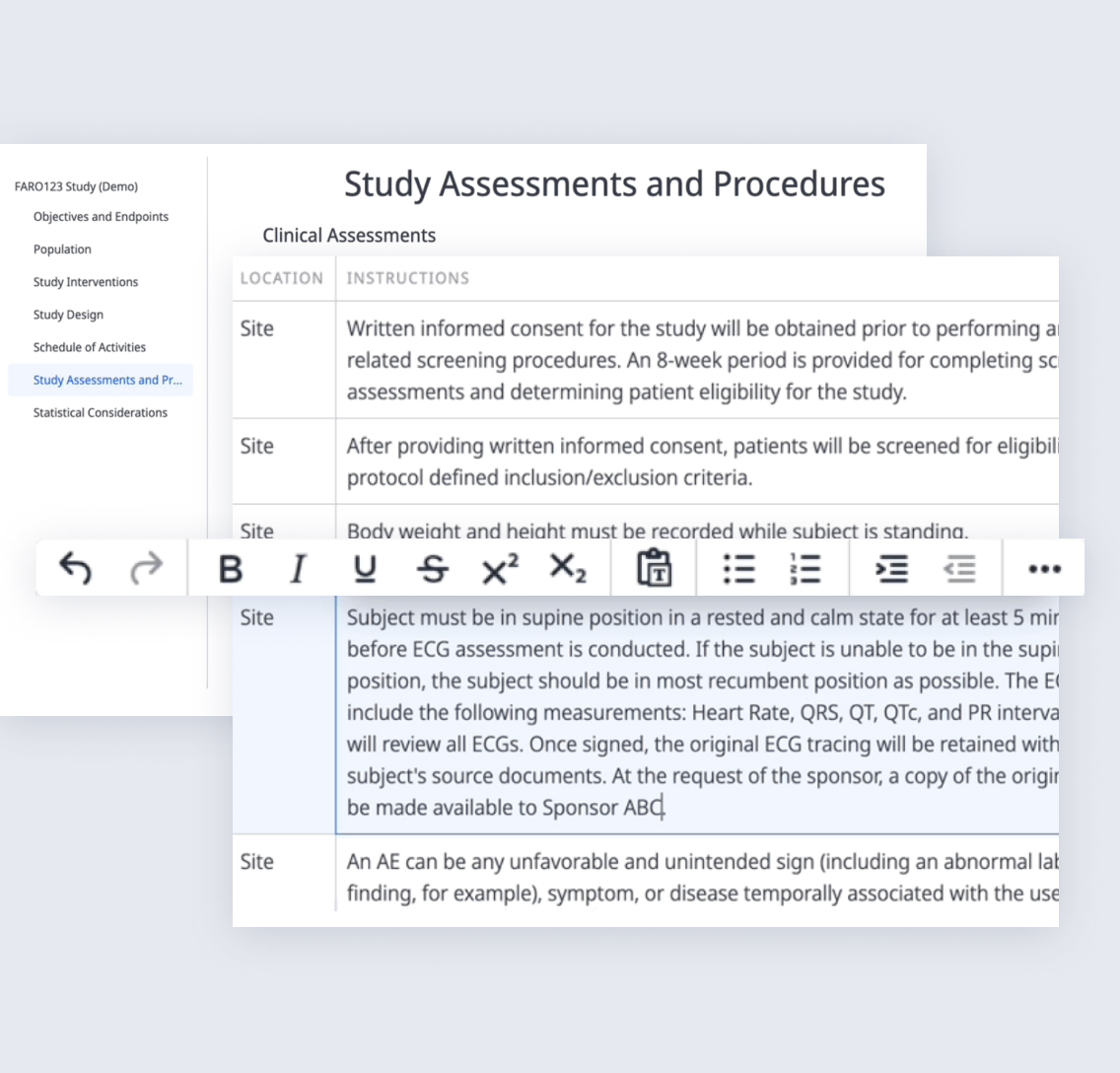
No more translating documents, clarifying details, or following up with stakeholders. When Clinical Data Managers receive a protocol designed with Faro, it’s already clear, structured, and optimized for swift startup.
Today’s clinical development landscape is more daunting than ever. To know their trials will succeed, sponsors need protocols that are built to deliver a new level of performance. For that, there’s Faro.
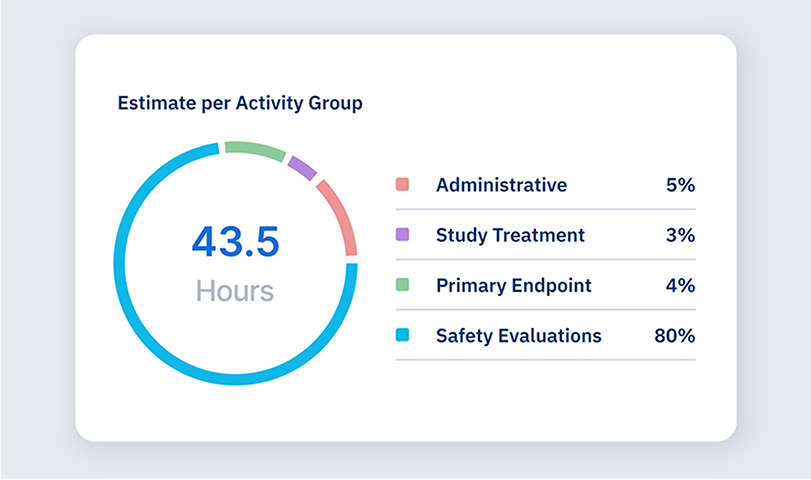

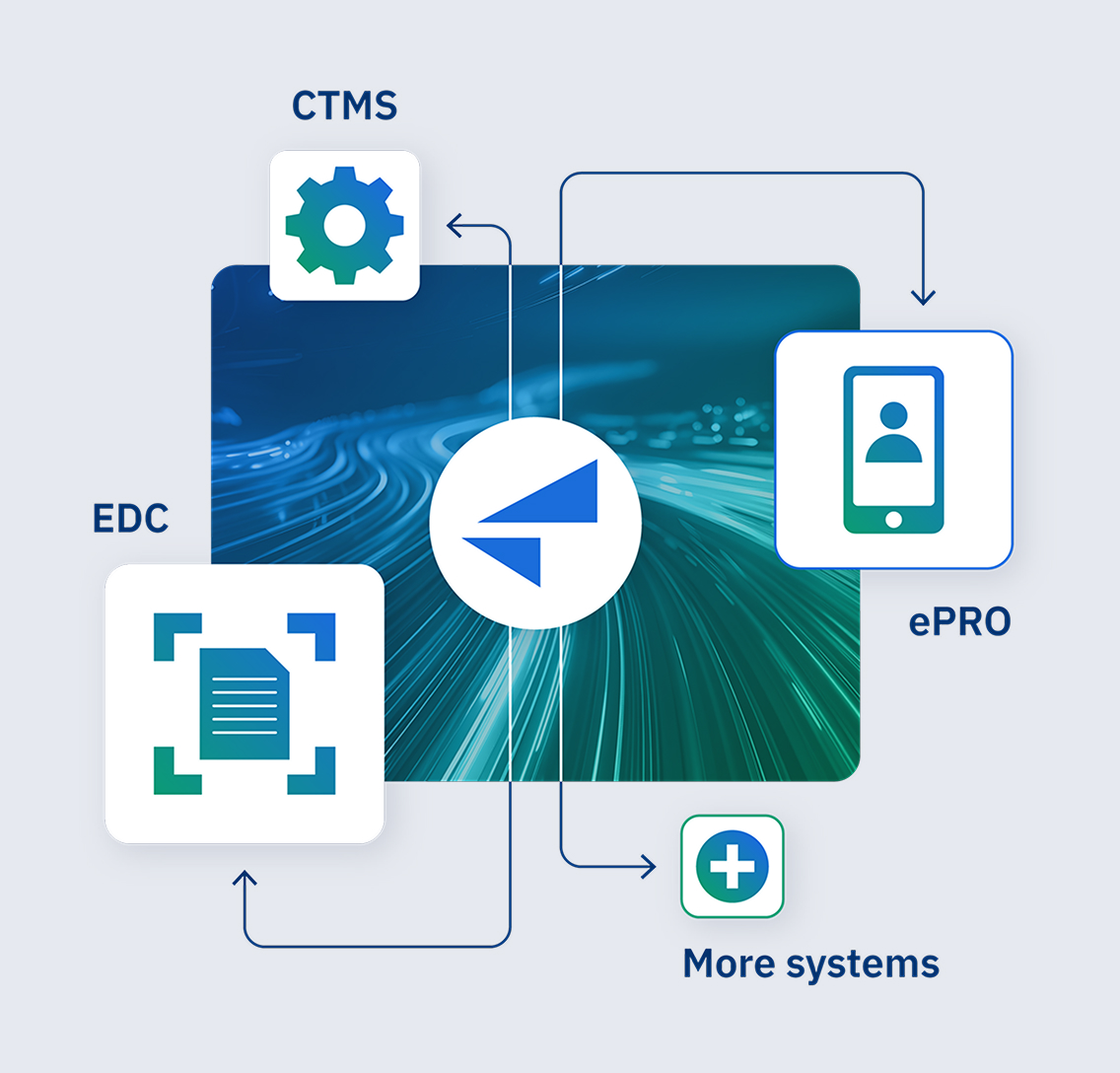
Forget time-consuming manual transfer tasks: Faro Study Designer integrates directly with the industry-standard EDC. Once your digital SoA is complete, you can send it to Veeva in a couple of clicks.

Empowered by generative AI
Faro’s industry-leading Study Designer comes equipped with the latest in AI-powered authoring tools. Together, our unique approach and best-in-class models help expedite documentation, ensure quality and accuracy, and deliver continual improvement.
Want to request a demo, explore our solutions, or learn more about our team? Submit our form and we’ll follow up in 1-2 business days.
All fields required.
A member of our team will follow up with you in 1-2 business days.