Explore cutting-edge generative tools that amplify experts’ skills, scale team capabilities, and accelerate innovation.
With cost and complexity growing exponentially, sponsors need groundbreaking new ways to expedite and de-risk their study programs. At Faro, we’re harnessing the power of AI to make those solutions possible
Our models are purpose-built for the complexities of clinical development, enriched with RWD, and localized to your program, enabling you to:

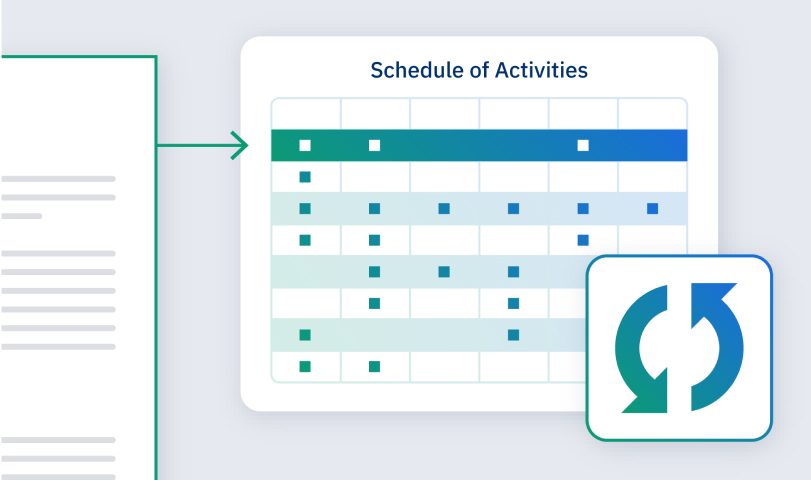
With the latest LLM-powered authoring technology built in, Faro Study Designer supercharges productivity while protecting quality and precision. Our AI Protocol Generator is fine-tuned by Faro’s clinical development experts and optimized for generating trial documentation.
When budgets, timelines, and patients lives are on the line, precision matters. That’s why our AI Protocol Generator is built for rigorous self-assessment and continuous improvement.


We never settle for off-the-shelf LLMs, general training data, or generic shared deployments. Our models are designed specifically for clinical development, tailored to your team’s needs, and fully secured for your use alone.
Every model we deploy is designed by data experts with deep knowledge of clinical development.

Our AI philosophy
As powerful as today’s LLMs may be, they’re no substitute for the skilled medical writers and experienced clinical professionals on your team. From checklist development, to prompt design, to final protocol, our AI’s outputs are guided, shaped, and validated by your experts—ensuring transparency, quality, and compliance at every step.
For Faro, powerful protocol generation solutions are only the beginning. Our technology bench is bursting with potential, with even more groundbreaking tools on the way soon.

Today

Coming soon
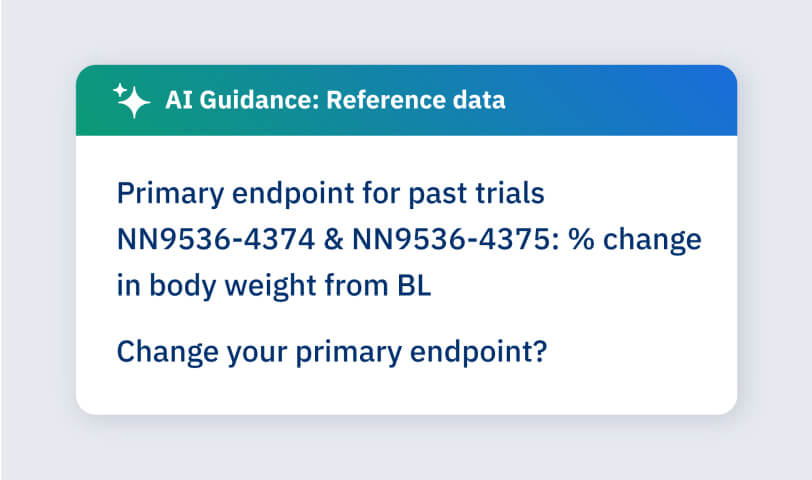
Coming soon
Want to request a demo, explore our solutions, or learn more about our team? Submit our form and we’ll follow up in 1-2 business days.
All fields required.
A member of our team will follow up with you in 1-2 business days.