The AI platform for accelerated clinical development
The world's leading biopharma teams design and execute better studies with Faro.


An end-to end platform for intelligent study design and execution
Trusted by industry leaders.
Proven ROI.
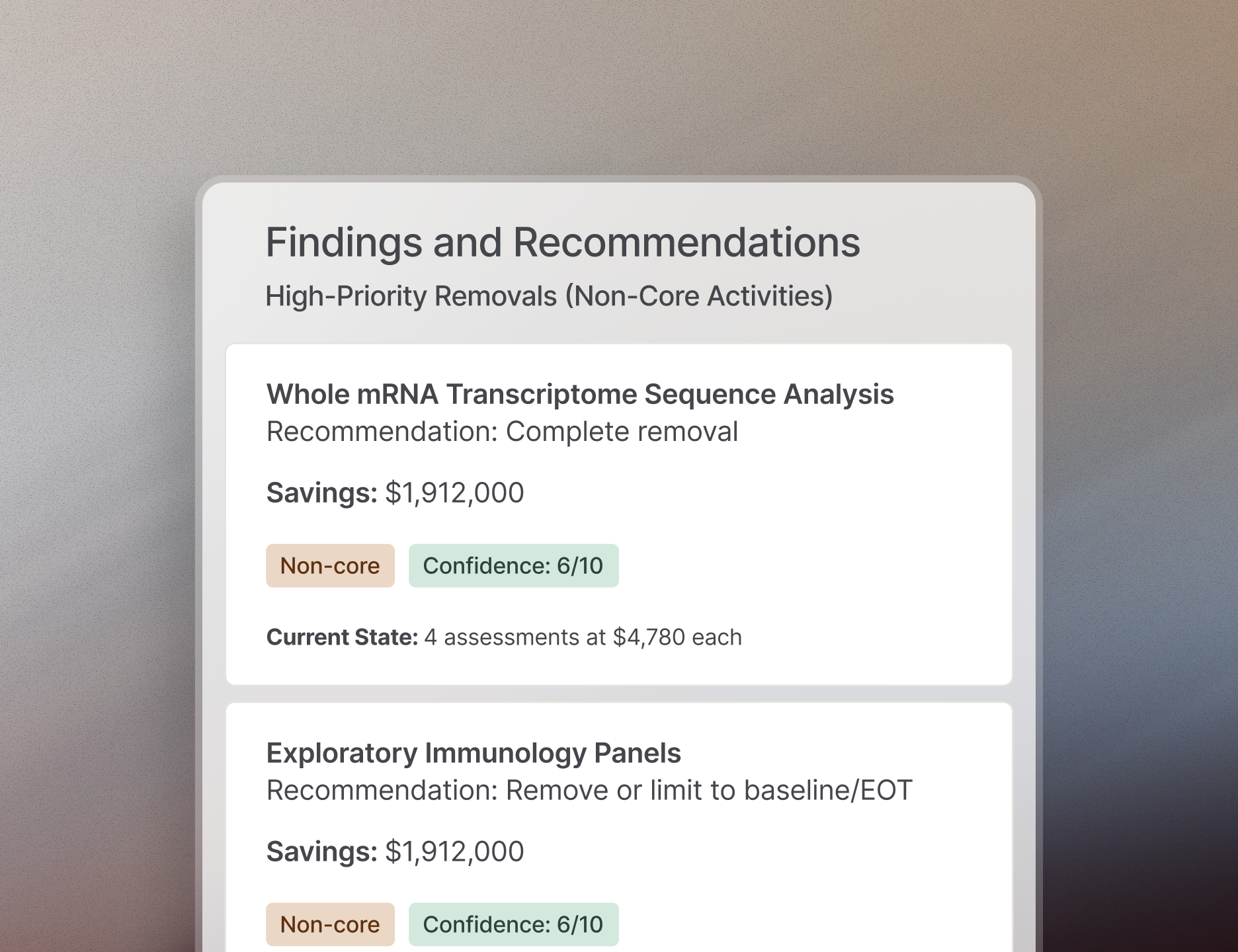
>$300 M
in potential cost savings identified
200 K
potential patient hours avoided
>1500
potential RVUs avoided

“We employed [Faro] to quantify the impacts of changes in an SoA to provide real-time feedback to the team and sponsor. The approach resulted in recommendations for substantial potential savings in participant and site staff time, costs, and complexity of the trials.”
Cummings SR, Chetham S, Lee A. A Method to Redesign and Simplify Schedules of Assessment and Quantify the Impacts. Applications to Merck Protocols. Ther Innov Regul Sci. 2024 Sep;58(5):789-795. doi: 10.1007/s43441-024-00666-x. Epub 2024 May 10